Why is your intuition about quantum spin incomplete?
|Difficulty level: Medium|
The pioneers and innovators have one thing in common. They have a clear intuitive picture of fundamentals of the subjects. In contrary fashion, the construction of intuition is not possible for all subjects. One such concept is quantum spin and most of young students and engineers have a sketchy picture of it.
Young students who do well in science and maths always try to come up with an intuitive picture of the concepts and theories, no matter how abstract the ideas are. Most of the science concepts are quite obscure, for example, non-dispersive wave equation given as
\( \frac{\partial^2\phi(x,t)}{\partial^2t}=v^2\frac{\partial^2\phi(x,t)}{\partial^2x}\)
As soon as student look at this equation, they build a mental picture as following- 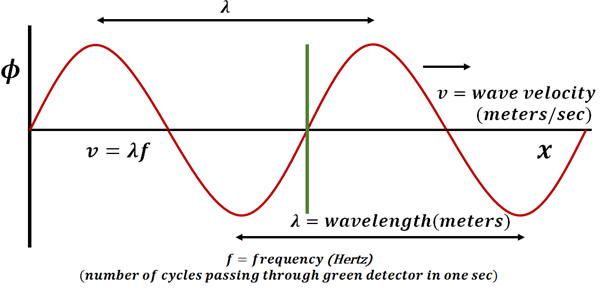 Students can build these pictures by comparing it with day to day experiences. For example, they create water waves by disturbing water reservoirs or produce waves by moving a rope up and down. But there are many other subjects in science like quantum mechanics where coming up with such mental pictures is not always possible. In the process of understanding the concept, students end up having incomplete intuition about it.
Students can build these pictures by comparing it with day to day experiences. For example, they create water waves by disturbing water reservoirs or produce waves by moving a rope up and down. But there are many other subjects in science like quantum mechanics where coming up with such mental pictures is not always possible. In the process of understanding the concept, students end up having incomplete intuition about it.
In this article, we will discuss one such concept of quantum mechanics known as quantum spin.
Do you remember whenever your teacher or some nerdy friend talk about spin? You always come up with a mental picture of either a spinning top or a planetary object or a ballet dancer. Because of this picture, most of the science and engineering students think of spin of elementary particles such as an electron and photon as some solid spherical object self-rotating about its axis. However, this picture is entirely heuristic and incomplete. 
Why is this so?
To understand this, let’s do some math. As we know from our earlier physics lectures, the angular momentum vector, \(\overrightarrow{S}\) is denoted by the cross product of particle position vector \(\overrightarrow{r}\) and its momentum vector \(\overrightarrow{p}\).
\(\overrightarrow{S}= \overrightarrow{r} \times \overrightarrow{p}= \overrightarrow{r}\times m \vec{v} = r_e m_e v \)
As we know from experiments like Stern Gerlach, the spin of an electron has magnitude \(\pm\hbar/2\) which is \(\pm\hbar/2=\pm0.525×10^{-34}\) joule sec, \(r_e\) is the Lorentz radius for electron which is interaction length of an electron given by 2.8 femtometers and \(m_e\) is the mass of an electron. When we put all this number, we end up with velocity approximately equivalent to 67c. This means the speed on the surface of the electron is more than 67 times the speed of light. Therefore the self-rotating picture of electron violates Einstein’s special theory of relativity, which states, “Nothing in-universe can move faster than the speed of light.” 
What do we understand from this?
The first, we cannot visualize electron as a point solid spherical particle. The second, spin is an intrinsic property of elementary particles like charge, mass, and spin. It can only be understood by relativistic quantum theory. There does not exist any classical analogous phenomena with the help of which we can visualize quantum spin. Richard Feynman once also wrote about this ambiguity: “It appears to be one of few places where there is a rule which can be stated very simply, but for which no one has found a simple and easy explanation. The explanation is deep down in relativistic quantum mechanics. This completely means that we do not have a complete understanding of fundamental principles involved”.
How do we know elementary particles possess these intrinsic properties if we cannot visualize or understand it?
There are many examples in science where scientists first observed some unexplained experimental results. They then tried to explain those result by formulating a theory. One such example is alpha decay, where Ernest Rutherford, in 1899, investigated alpha particles, and by 1928, George Gamow using quantum tunnelling, formulated the theory of alpha decay. Similar to the concept of quantum spin, we cannot find the same daily life experience of quantum tunnelling. But we do know it exists by detection of alpha particles. The observation that two electrons repel each other leads us to believe that charge exists. The observation that the nucleus of hydrogen (proton) is 1835 times heavier than electrons gives evidence about the constant mass of an electron.
Finally, there are experiments like Stern Gerlach and Zeeman effects which support the theories of quantum spin. If you find this article interesting and also want to visualize complex science ideas with animations and videos. Then also visit our youtube channel ‘Physics Gyata‘ and subscribe.
Please also share this article and subscribe to Quantuse Newsletters for such awesome science stuff.
Disclaimer: If there is something bothering you about the content of this page kindly visit the disclaimer page by clicking here.
©Quantuse




